Risk Evaluation and Education of Alzheimer's Disease: the Study of Communicating Amyloid Neuroimaging

Alzheimer’s disease (AD) clinical trials have traditionally tested individuals with symptoms of dementia, but the discovery of AD biomarkers has dramatically altered this approach. New studies are enrolling participants who are cognitively normal but have biomarkers suggestive of “preclinical AD” with the hopes of delaying the onset of cognitive impairment. Of critical importance is whether individuals’ knowledge of their biomarker status biases cognitive outcomes. If simply learning that one is biomarker positive causes a person to perform worse on cognitive testing, then primary outcomes data of AD trials may not be valid. Additionally, there are questions about whether people change their behaviors after learning their biomarker status and whether providing the information causes psychological and social harm.
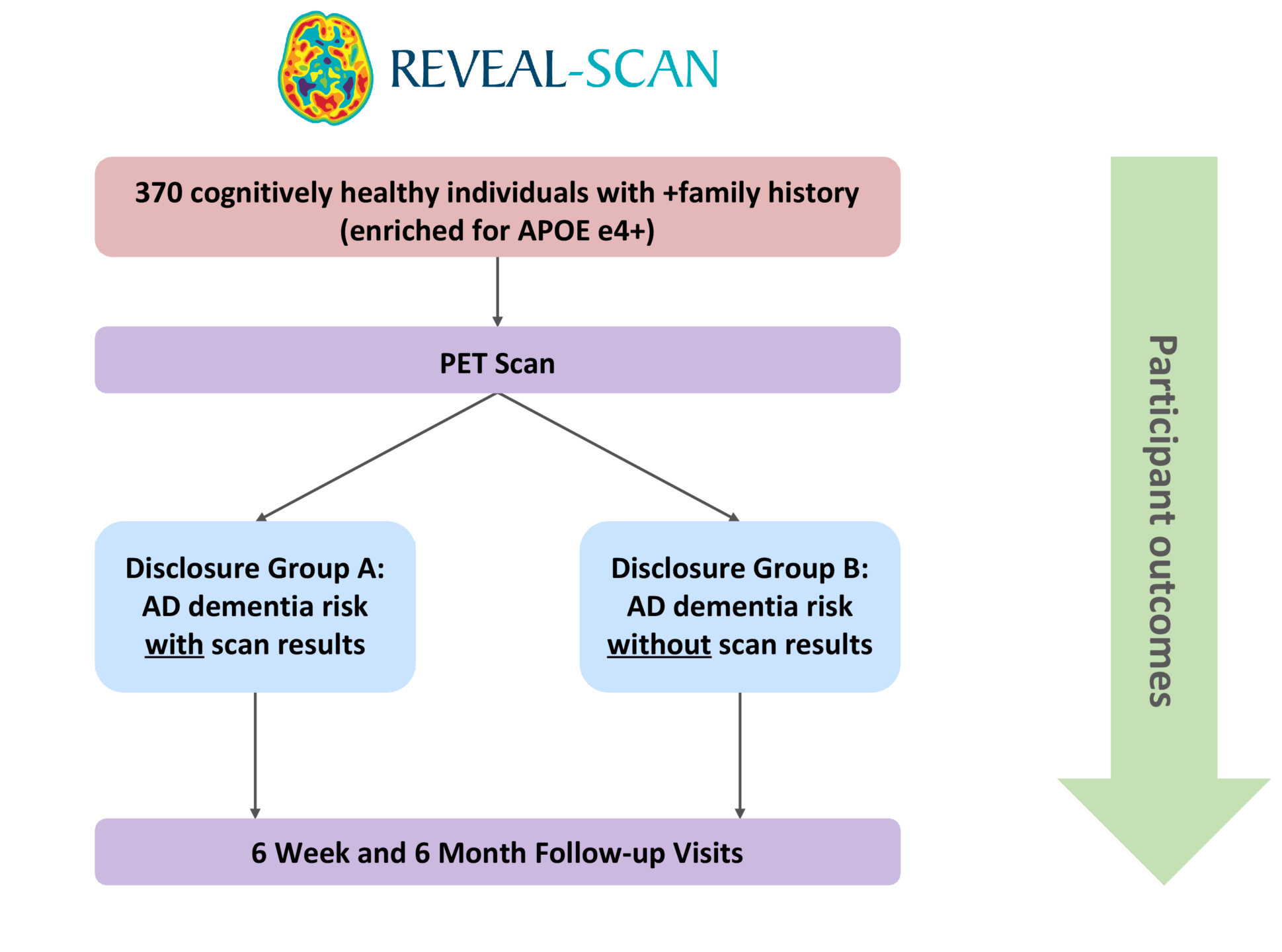
The REVEAL-SCAN (Risk Evaluation and Education of Alzheimer’s Disease: the Study of Communicating Amyloid Neuroimaging) Study, funded by the NIH, is the first multi-site, randomized clinical trial to examine the impact of learning amyloid imaging results in cognitively normal individuals. In this study, protocols were developed to communicate results from amyloid PET brain imaging. Currently, we are enrolling 270 cognitively normal individuals, with an effort to enroll 25% minority populations, between the ages of 65 and 80. Participants are randomized to receive their amyloid scan results or not, and then followed for a period of 6 months.
 The aims of REVEAL-SCAN are 1) to determine whether disclosure of elevated brain amyloid will bias ADCS-Preclinical Alzheimer Cognitive Composite test results; 2) to determine whether disclosure of elevated brain amyloid will cause psychological distress; and 3) to explore how learning amyloid imaging disclosure will impact preventative health behaviors, advance planning for health and well-being.
The aims of REVEAL-SCAN are 1) to determine whether disclosure of elevated brain amyloid will bias ADCS-Preclinical Alzheimer Cognitive Composite test results; 2) to determine whether disclosure of elevated brain amyloid will cause psychological distress; and 3) to explore how learning amyloid imaging disclosure will impact preventative health behaviors, advance planning for health and well-being.
For more detailed information about the REVEAL-SCAN Project, including recruitment and eligibility details, study design and outcome measures, view this handout, call us at 617-264-5876, or visit our clinical trial page:
REVEAL-SCAN on ClinicalTrials.gov
For scientific papers and media coverage, or to donate to our REVEAL-SCAN Project, visit the links below or email us at revealscan@partners.org.








